Oncogenic RAS mutants activate
the RAC/CDC42-dependent kinase PAK1 through a few distinct (and independent) signalling
pathways. One is the RAS-PI3 kinase-RAC/CDC42 pathway. A second one is RAS-ETK
pathway in which RAS activates ETK gene expression. A third one is RAS-FYN
pathway in which RAS activates FYN gene expression. The first pathway can be
blocked by CAPE in propolis, which down-regulates RAC. The second pathway can
be blocked by the ETK inhibitor AG 879 which interferes with ETK-PAK1
interaction. The last pathway can be blocked by a series of FYN inhibitors such
as PP1/PP2 and SU6656. I have previously
described how FYN could activate PAK1 through a tumour suppressor called HT1. HT1
is a direct inhibitor of PAK1, and FYN phosphorylates and inactivates HT1.
Recently, Claire Bastie’s
group at Albert Einstein College of Medicine in NY found another FYN pathway
leading to activation of PAK1 (1). They noticed first that FYN-deficient mice
have a less fatty (lean) mass than the control normal mice, and in both
skeletal muscle and adipose tissue of the former, the higher kinase activity of
AMPK, than the latter. Treatment of the control mice with the FYN-inhibitor
SU6656 also did lead to a loss of fat and activation of AMPK. Then they found
that FYN directly phosphorylates LKB1, the major AMPK activator, at Tyr 261 and
365, for the inactivation, and blocking FYN results in the export of LKB1 into
cytoplasm (from nucleus) and activation of AMPK. This indicates that FYN inactivates
directly LKB1.
Since LKB1 is known to
inactivate PAK1 and activates AMPK simultaneously, it is now clear that FYN can
activate PAK1 by inactivating the two distinct tumour suppressors, LKB1 and HT1.
In other words FYN inhibitors would be useful not only for cancer therapy, but
also for the treatment of type 2 diabetes and obesity.
Reference:
- Yamada E, Pessin JE, Kurland IJ, Schwartz GJ, Bastie CC. Fyn-dependent regulation of energy expenditure and body weight is mediated by tyrosine phosphorylation of LKB1. Cell Metab. 2010 11:113-24.










































































![リオ五輪男子体操団体:日本(金)、ロシア[銀]、中国[銅]。](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEjHS61FORcH43CteZVfJzLmbqvNwOIliOSMpTpRtEi7x8j1ZwPk5rDaZovTrwuZxfDDtdEDSj673it735LF0mweIunaj7ja07lURBDYTV6wPMaAlumFt3aWWzYbHZgIaxcOLk_OKEMyQ3lX/s1600/2016+taiso+gold.jpg)
![皇太子(明仁)による沖縄訪問 [1975年]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEjvSQrzV7yw_4gVQSwxZP_jh4VnEJscSqOqbiBh0VdAK3CRddXRqkd70JdLyws9fGejk-FGVmXWbHvSxlF3f8UogTyf9KXbqU1NGXesvcx2Hlsd6uq81AHweeioc61wynq3d2IYuyolijgT/s1600/akihito+message.jpg)


















































































































































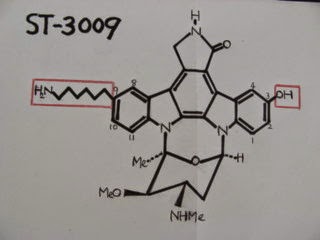







































![アルニカ [ウサギ菊]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEilqv0qou-4NpoUh1PFWYK0FSaozKazee0VYGxsFtfjBma46ya9yxqB6X9Ziuob25tNRpBbnFIcUFlOEjz1WcAjVNzjGl1E-QbDgE7VOLkjZDx0eplJ1WJHf0fTEWXxf8F5G-cHUhqHELY9/s1600/ArnicaS.jpg)








































































































































.jpg)


















































































.jpg)
















































0 件のコメント:
コメントを投稿